
It couldn’t be more obvious. The fry are ready to leave the nest. The trout in our virtual trout tank at the Trailside Museum are very active, hungry and ready for more space. Today we lowered one corner of the basket to let the brave fish swim out and search for food.
The increase in the metabolic process in the tank leads to ammonia buildup and kicks off the nitrogen cycle. This cycle relies on microscopic, but hugely important, nitrifying bacteria to convert the toxic ammonia to nitrite and then nitrate.
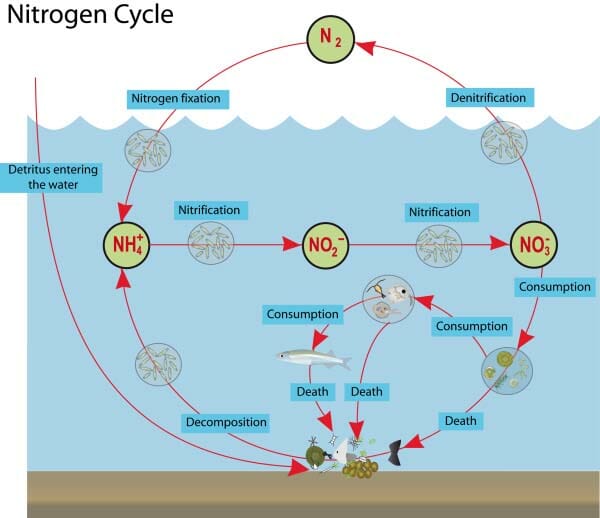
Ammonia/ ammonium (NH3 and NH⁺ ₄) are excreted by the trout and also form from the breakdown of uneaten food. Ammonia is toxic to trout and can cause damage to their delicate gills.
Nitrite (NO2−) forms by bacteria that consume the ammonia. Nitrite, too, is harmful to trout, even in small amounts.
Nitrate (NO3-) forms by bacteria that consume nitrite. Nitrate is only harmful when it is present in very high concentrations and can also be converted to Nitrogen gas by bacteria. Nitrate is an important nutrient source for plants.

To help the process along, we add bacteria to our trout tank and monitor water quality bi-weekly. Using the API Freshwater Master Test Kit this week, we tested tank ammonia, nitrite and nitrate. Our results weren’t great although, the small fry seem to be thriving. Hopefully the 20 percent water change and some additional bacteria will get our tank cycling properly. We will be back to check things out again later in the week.
The aquatic nitrogen cycle relies on bacteria and decomposers to create a safe and non-toxic environment.
Science Journal Question for TIC Students: What are the parts of a riparian stream ecosystem that help fuel the nitrogen cycle. How do trees help trout and how do trout help the trees?
Dive Even Deeper: What are the components needed to write a balanced equation for the conversion from ammonia (NH3) to nitrite (NO2−)? How about for nitrite (NO2−) to nitrate (NO3-)? And nitrate (NO3-) to nitrogen gas (N2)?



